Strategy
Rather than focus on a single product or indication, the larger opportunity lies in building a robust and diverse product pipeline of high-value potential drug candidates to treat psychiatric illnesses. We are an Intellectual Property (IP) Centric Company. IP is at the center of everything we do. We believe it is management's responsibility to invest tens of millions of dollars to develop product candidates that have a strong, proven intellectual property base.
Development strategy
Partnering is a key part of our strategy and our team of drug hunters is continuously on the lookout for innovative technologies and assets with strong patents. We take a broad, multi-pronged approach to identify drugs that can be repurposed as drug candidates for psychiatric disorders.
Our pipeline development strategy reflects a blended approach to manage risk and catalyze partnerships that enable access to non-dilutive capital, and increase speed.
Lead Product Candidate
 LMI-107, together with its proprietary dosage form, will be developed to meet the requirements of women undergoing cancer therapy to address vasomotor symptoms (VMS), indicated by debilitating hot flashes and night sweats. As there is no FDA-approved treatment for emergent VMS in cancer therapy, LMI-107 may represent a highly valuable asset for Big Pharma partners. The product profile is uniquely differentiated and positioned to help a vulnerable population. Beyond VMS, caused by endocrine therapy, cluster analysis shows that pain and depression are often present, driving many women to discontinue potentially life-saving medications.
LMI-107, together with its proprietary dosage form, will be developed to meet the requirements of women undergoing cancer therapy to address vasomotor symptoms (VMS), indicated by debilitating hot flashes and night sweats. As there is no FDA-approved treatment for emergent VMS in cancer therapy, LMI-107 may represent a highly valuable asset for Big Pharma partners. The product profile is uniquely differentiated and positioned to help a vulnerable population. Beyond VMS, caused by endocrine therapy, cluster analysis shows that pain and depression are often present, driving many women to discontinue potentially life-saving medications.
THE PROBLEM
Approximately 1 million women in the United States are currently living with early-stage, hormone receptor-positive (HR+) breast cancer—the most common subtype of the disease. Standard treatment involves long-term use of aromatase inhibitors (AIs), which reduce estrogen levels and lower recurrence risk by 40–50%. However, estrogen suppression frequently causes vasomotor symptoms (VMS), including severe hot flashes and night sweats, as well as depression, fatigue, and pain. These side effects are among the most common and disruptive consequences of endocrine therapy.
CURRENT TREATMENT APPROACH
Although guidelines recommend 5 to 10 years of AI therapy, real-world adherence is poor. Over 250,000 women have discontinued treatment due to intolerability. Of the approximately 195,000 women who initiate endocrine therapy each year, about 25%—nearly 49,000—stop within the first year, primarily due to VMS and depression. This represents a critical point of failure in the treatment pathway. Discontinuation is associated with a 20–30% increase in recurrence risk, and many of these recurrences present as stage IV metastatic disease.
THE LMI ANSWER
LMI-107, the lead clinical candidate from Luminous Mind Inc., is designed to address the primary tolerability barriers to endocrine therapy: VMS and comorbid depression. It has shown potential to improve both domains without the drawbacks of existing treatments. LMI-107 is energizing without being anxiogenic, and it does not cause weight gain, sexual dysfunction, or cognitive impairment—side effects commonly associated with SSRIs and SNRIs.
By enabling women to stay on their prescribed endocrine therapy, LMI-107 has the potential to reduce preventable recurrences and improve survival, while also enhancing quality of life during treatment.
Team History - Repurposing
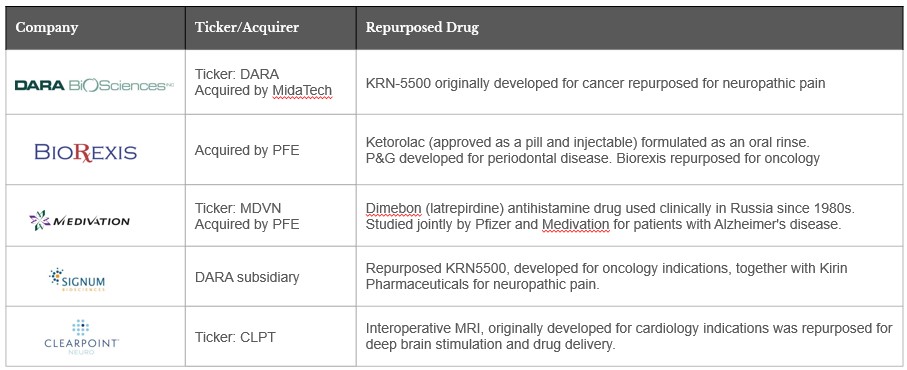
Drug repurposing refers to the process of identifying new indications for existing FDA-approved drugs, product candidates under development, and abandoned compounds. The advantage of repurposing is that it significantly reduces the time required for development since the safety of the compound has often been tested. In fact, approximately 30 percent of repurposed drugs ultimately reach patients, which is a substantial improvement compared to the 10 percent success rate of the traditional drug development process.
Our team has successfully worked together to repurpose drugs and devices in several other successful companies like these below and has created significant value for patients and stakeholders.
In-Licensing Drug Programs

Often, pharmaceutical and biotech companies decide to curtail programs that are no longer a priority due to business decisions and not for scientific reasons. Many of these programs are fairly advanced, from a development prospective, where much of the early clinical work has been performed, and the drug is ready for further clinical testing.
In the current financial environment, in-licensing opportunities offer additional advantages to companies who need to focus on their primary assets, while maintaining an investment in their original work. This offers us a unique situation to in-license these advanced assets.
We are able to move quickly to a clinically viable drug, which provides a substantial cost savings. This strategy has the advantage of de-risking the early stages of drug discovery and development, which ultimately will lead to a more rapid timeline to achieving a ROI.
Artificial intelligence (AI)
Drug repurposing depends on massive observed data from existing drugs and diseases. We utilize AI approaches for the systematic identification of drug repurposing leads based on big data resources.
AI plays a crucial role in providing researchers with tools to model and navigate the biological networks in which drug targets reside. AI of various architectures, themselves inspired by neural networks, learn and expose the implicit rules that govern the performance of biological pathways in health and disease. AI also provides us with predictions of previously unknown connections identifying successful new drug-target combinations.
pharmaceutical patents

We seek "composition of matter" patents, also called product patents – the strongest possible type of patent which is not circumventable. This is what our substantial partners require.
We are not proponents of formulation, crystalline forms, and process patents. Our product candidates meet the stringent criteria for partnering with Big Pharma.
